Connected IVD Solutions, made simple
Jump on the
digital, cloud-based, IVD train
without the hassle of building the entire backend of the application yourself with the Extra Horizon Medical Backend-as-a-Service.

INTRODUCING EXTRA HORIZON
Your go-to medical Backend-as-a-Service platform for building connected IVD solutions
Research Phase
Turn your idea into a prototype while performing all the necessary research. You will develop a comprehensive, working prototype with the help of the Extra Horizon development cluster.
Pre-market Phase
Build your first true digital medical product to pass the clinical validation stage. We provide you with all the needed infrastructure to comply with the medical security and privacy standards.
Commercial Phase
Take the next steps to bring your medical device to the market and beyond by getting your CE, FDA, ... mark. Extra Horizon is ISO13485 certified, enabling you to meet the regulatory obligations with ease.
THE BENEFITS FOR MEDICAL IVD COMPANIES
What our platform can do for your digital IVD device roadmap
If you thought building a digital IVD app was difficult, wait until you learn about the regulatory demands (but you are probably already aware of this). A key aspect of medical software development is the oh-so stringent regulatory roadmap your company and product will need to go through in order to successfully launch into the market.
Being ISO 13485:2016 compliant and certified as a company is the gold standard in digital IVD software development. Extra Horizon is the trusted supplier that fits perfectly with your company. Not only is our platform built and documented according to the IEC 62304:2006 standards, our company is also certified against all necessary Security and Privacy regulations (GDPR, HIPAA, ISO27001, ISO 27701, EU Cloud CoC and so on).
Building a digital IVD solution from scratch takes time, effort, and way too many sleepless nights. With Extra Horizon, you can start configuring right away. No need to write every single piece of code. You will have a working prototype in a matter of weeks or even a market-ready application in a matter of months.
In essence, most digital health solutions have a lot in common. They will need to add an algorithm, perform user authentication, create reports, send emails, ping notifications, provide gateways and so on. Extra Horizon enables you to use only the services you need at any point in time. Also, your developers will love working with our well-documented SDK, making their work even more enjoyable.
For a full list of the services included in our platform, click here. Want to know how it works? Then be sure to have a look at our developer documentation here.
The unique aspect of our medical Backend-as-a-Service platform does not solely lay solely in the technical or regulatory aspects. It’s the combination of both that will enable you to start building right away, in line with all the audits you will need to pass somewhere down the line. And all this by a supplier you can trust completely.
You will also need a much smaller team to complete your journey, and will also be met with subscription packages that scale only when your IVD product scales. Using a tried and tested platform will save you from all the unforeseen costs you will encounter when building the solution from scratch.
Save time, save costs & launch quicker, with the Extra Horizon medical Backend-as-Service platform.
AN IMPORTANT CHOICE TO MAKE
To build, or buy, your IVD solution's backend infrastructure
You have an idea for a new digital IVD product, that includes (or is a) SaMD (Software as a Medical Device). Great!
Now the big question surfaces: will you build your cloud-based backend infrastructure completely from scratch, or will you build it on an
existing, flexible and scalable backend platform like our own?
BUILDING A CE-MARKED IVD SOLUTION
The long road to obtaining the CE Mark for your IVD solution

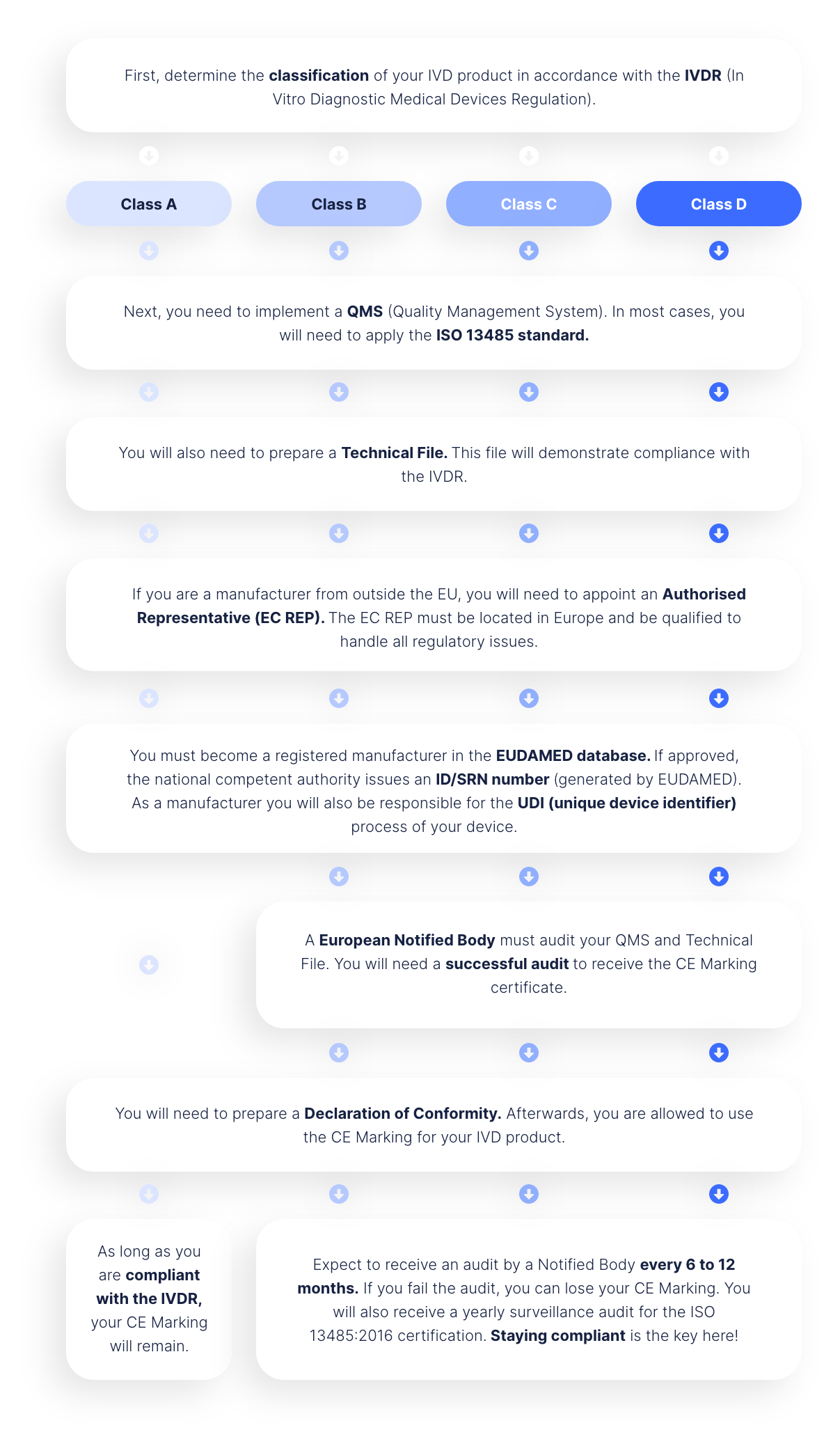
First, determine the classification of your IVD product in accordance with the IVDR (In Vitro Diagnostic Medical Devices Regulation).

Next, you need to implement a QMS (Quality Management System). In most cases, you will need to apply the ISO 13485 standard.

You will also need to prepare a Technical File. This file will demonstrate compliance with the IVDR.

If you are a manufacturer from outside the EU, you will need to appoint an Authorised Representative (EC REP). The EC REP must be located in Europe and be qualified to handle all regulatory issues.

You must become a registered manufacturer in the EUDAMED database. If approved, the national competent authority issues an ID/SRN number (generated by EUDAMED). As a manufacturer you will also be responsible for the UDI (unique device identifier) process of your device.

You will need to prepare a Declaration of Conformity. Afterwards, you are allowed to use the CE Marking for your IVD product.

Expect to receive an audit by a Notified Body every 6 to 12 months. If you fail the audit, you can lose your CE Marking. You will also receive a yearly surveillance audit for the ISO 13485:2016 certification. Staying compliant is the key here!

A European Notified Body must audit your QMS and Technical File. You will need a successful audit to receive the CE Marking certificate.

As long as you are compliant with the IVDR, your CE Marking will remain.

Class A

Class B

Class C

Class D
Having trouble reading this figure? Try it out on tablet, laptop or dekstop instead!
Feeling a bit overwhelmed? We are here to help.
Not sure if Extra Horizon can support your IVD solution?
That’s the beauty of it. The Extra Horizon medical Backend-as-a-Service forms the foundation of your solution. We offer you the tools to start building the SaMD solution of your dreams in no time.
We are here to
support your idea. From connecting biosensors, to blood tests, stool analysis, home testing, genetic screening, diagnostic services, questionnaires, self assessment tests, DNA tests, COVID-19 tests and so on.
Some typical solutions we support ↓
Companion solution and digital IFU
Companion solution with result logging & education
Digital registration solution of LFT results and analysing and/or sharing with HCP's
Connected solution between mobile phone and sensors for read-out and result display
Companion solution and digital IFU
Companion solution with result logging & education
Digital registration solution of LFT results and analysing and/or sharing with HCP's
Connected solution between mobile phone and sensors for read-out and result display
Still in need of funding?
Most new digital health initiatives require some kind of investment. For larger, established companies these investments more often than not come from internal funds. But for startups and SMEs, most often you will require outside funding from investors.
So what are these investors looking for, and how can building on Extra Horizon help you
convince them of your IVD breakthrough?
Experience
By working with a trusted supplier, experienced in launching SaMD, you’ll have the right foundation needed to no time.
Scale
International regulatory compliance and high volume throughput solutions require specific cloud infrastructures.
Speed
Your competition is moving fast and state-of-the-art solutions are already having regulatory approvals, your time to market is key.
Secure data
Handling sensitive data, data sharing and obtaining insights requires GDPR and HIPAA requirements.
Talk to us today
Solutions
BY USE CASE
BY CAPABILITY
BY STAGE
Getting Started
AS A DEVELOPER
AS A PARTNER
© 2023 Extra Horizon, All rights reserved
Kempische Steenweg 303, 3500, Hasselt, BE
— Hasselt, Belgium

